Bookshelf
Assessing Cement Plant Thermal Performance
John Kline (Principal), Charles Kline (Researcher)
Kline Consulting
I. INTRODUCTION
Experience has shown that there are just a few elements in Portland cement manufacture that need to be understood in order to assess a manufacturing facility’s thermal performance. This paper will review the basics of thermal performance assessment and explain why and how they can be used.
Portland cement is a manufactured product that is made up primarily of calcium silicate crystals. The calcium usually comes from limestone while the silicates can come from sand, clay, or shale. The raw materials are heated to the reaction temperatures in a preheater. The calcium silicates are fused together at high temperatures in a rotary kiln to form an intermediate product referred to as “clinker”. The clinker exits the rotary kiln at a high temperature and is cooled in a device known as the “clinker cooler”. The cooled clinker is ground into a fine powder to produce Portland cement.
Thermal efficiency is the relationship between the heat required to drive the chemical reactions and the overall heat consumption of the process. The heat consumption of the process is the total amount of heat required from the fuel to produce the product. Heat consumption is often expressed as thermal units per unit weight of product, such as million BTUs / short ton, kCal/kg, or GJ/kg. Clinker is the product most often referenced for thermal efficiency in Portland cement manufacture.
The industry normally considers the heat consumption to be only that heat required in the clinkering process. Any additional fuel that may be required for material drying, etc. is often excluded when benchmarking facilities. As the exhaust gases leave the process above the steam condensation temperature, the heat consumption is normally based on the lower heating value of the fuel(s) being used.
The starting point of a thermal assessment is understanding the characteristics of the raw materials and their impact on the prepared kiln feed and product. This analysis allows for an estimation of the fundamental heat required to drive the necessary chemical reactions. This sets the bare minimum requirements for producing clinker. The heat is supplied by combusting fuel in the reaction chambers known as the kiln and precalciner. A large amount of heat leaves the reaction chambers in the sensible heat of the clinker produced and the combustion exhaust gases.
The preheater recuperates heat from the combustion gases leaving the kiln and precalciner. Likewise, the clinker cooler recuperates heat from the clinker as it exits the kiln. The effectiveness of the heat recuperation in both the preheater and cooler has the most direct impact on the process heat requirements. Therefore, the fundamentals of the system thermal efficiency are fixed by:
- the characteristics of the raw mix;
- the recuperation effectiveness of the preheater;
- and the recuperation effectiveness of the cooler.
Heat loss to the environment due to radiation from the equipment is often less than 10 % of the total heat consumption. The amount of heat loss due to radiation is fixed by the equipment design. This heat loss is considered a constant for an operating plant and not addressed here.
Having established the basics for system thermal efficiency, the assessor now needs to look at system stability. Instability is the third largest controllable cause of poor thermal efficiency after preheater and cooler operation. Process instability comes from a lack of consistency in the quantity and quality of the raw mix and fuel. Mechanical instability comes from breakdowns in equipment often requiring cool down and reheat periods. Mechanical instability is not addressed here as it does not impact steady state conditions.
Understanding these basic principles allows for a quick remote or on-site assessment of thermal performance and provides insights to the steps required to improve it. Maximizing thermal recuperation and system stability will deliver the best thermal efficiency and a sustainable competitive advantage.
II. RAW MATERIAL CHARACTERISTICS
The chemical and physical characteristics of the raw materials set the stage for the thermal requirements. The heats of reaction are set by the quantities of the various minerals in the raw materials. The reaction temperature is set by both the quantities and physical properties of the raw materials as fed to the process. The combination of required heats of reaction and the reaction temperature determine the base amount of heat required and determine the starting points for heat recuperation in the preheater and cooler.
A. Chemical Characteristics
The basic formula for Portland cement has been fixed by specifications and is generally universally applied, even if specifications do vary region by region. Portland cement clinker is made up of calcium, aluminum, silicon, and iron oxides which are recombined to form calcium silicates, calcium aluminates, and calcium aluminoferrites as seen in table I. The raw materials that provide the necessary mineral oxides are primarily limestone, clay or shale, sand, and iron ore or oxide.
Table I
Portland cement Raw Materials and Clinker Compounds

The fundamental ingredient and primary driver of the heat required for the chemical reactions is the amount of calcium carbonate (CaCO3) used. The calcium carbonate typically comes from limestone and needs to be activated in order to combine with the other minerals. Calcium carbonate is activated by calcining, or driving off, the carbon dioxide and converting the limestone to lime. The limestone calcination reaction is highly endothermic, meaning that it requires a lot of heat to drive the reaction that releases the CO2. This heat requirement is + 3200 kJ/kg and typically accounts for the majority of the heat required for all the chemical reactions required to make clinker.
CaCO3 ↔CaO+CO2 ∆H=+3200kJ/kg (1)
Table I demonstrates that the limestone contributes lime (CaO) to all four of the clinker compounds. One common measure in the cement industry is known as the lime saturation factor (LSF). This factor estimates whether there is sufficient lime in the raw mix to produce all of the clinker compounds. The lime saturation factor calculation is shown in (2) below. Target values are plant specific, but typically around 100 %.
LSF = %CaO / (2.8 * %SiO2 + 1.18 * %Al2O3 + 0.65 * % Fe2O3) (2)
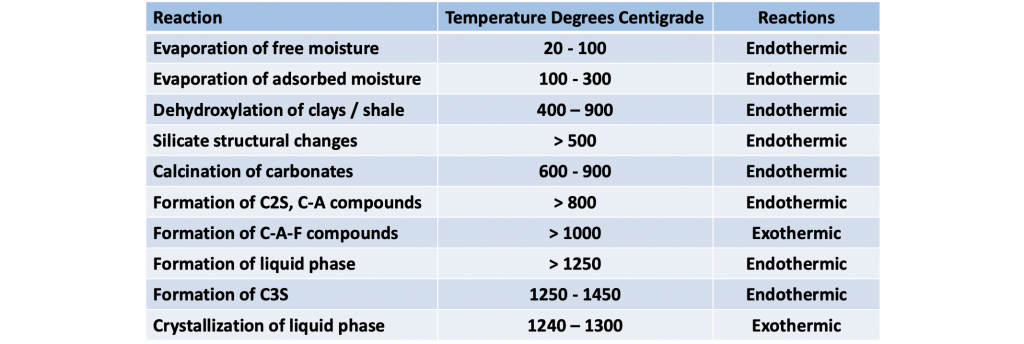
Other reactions requiring minor amounts of heat include the drying of the raw material and the release of combined water from the clay and shale components. Further heating breaks down the silicate structures in the clays and shale. Finally, the various minerals are combined into the new compounds in a series of endothermic and exothermic reactions. These heat requirements are also rather insignificant and typically account for less than 10 % of the total heat required for all chemical reactions. A summary of the typical chemical reactions and the corresponding temperature ranges is provided in table II.
Table II
Portland cement reactions and their approximate temperature ranges
The heat of reaction (HR) in kJ/kg-clinker can be estimated from the chemical composition of the clinker as shown in (3) using the weight fractions of the different oxides. It should be noted that the contribution of the alumina (Al2O3) component can vary depending on the quantity of combined water in the clay. Different clay types have different amounts of combined water. The Al2O3 value used in (3) is the most common one.
HR =3200*CaO+2710*MgO–2140*SiO2 –250*Fe2O3 +1720*Al2O3 (3)
The heat of reaction is fixed by the chemical composition of the raw materials used to make the clinker. The chemical composition of clinker is normally controlled by specifications and is fairly uniform across the globe and does not normally change within a given facility. The heat of reaction can be estimated at 1750 kJ/kg-clinker if the precise chemical composition is unknown.
B. Physical Characteristics
The raw materials need to be of a certain fineness in order to intermix and react properly. Quarried limestone often starts out in large boulders as much as one meter in diameter. The material being processed typically needs to be at 90 % less than 90 microns in size. The size reduction is achieved through a combination of crushers and mills. In modern plants, an impact crusher is often used for single pass primary crushing in combination with a vertical roller mill for fine grinding. In older plants, ball mills were utilized for the raw material grinding.
In general, the finer the raw mix, the easier the material will combine to form the clinker compounds. This does not change the heat of reaction, but will impact the temperatures required to drive the reactions. Limestone particles larger than 100 microns are deemed unacceptable as the lime (CaO) in the interior of the particle may not combine with the other mineral oxides.
As seen above, the SiO2 portion can come from clays, shale, and / or sand. When the silica is in the form of quartz, it can be problematic. Quartz is a hard material to grind and can often be seen concentrated in the coarser fractions of the raw mix. When coarse quartz (> 45 microns) is present, the material needs to be heated to a higher temperature and held longer at that temperature to allow the silica to combine.
One of the issues that exists in fine grinding is that the softer materials tend to be over-ground (or too fine) while the harder materials are under-ground (or too coarse). This is especially true in vertical roller mills. Figure 1 demonstrates the concentration of coarse quartz (red dashed line) in the coarser fractions of the kiln feed from one cement plant. Ideally, the lime saturation factor should be around 100 % for all size fractions. The blue bars indicate that very little limestone (the softer material) is contained in the coarser size fractions, therefore this plant will require a higher reaction temperature than normal.
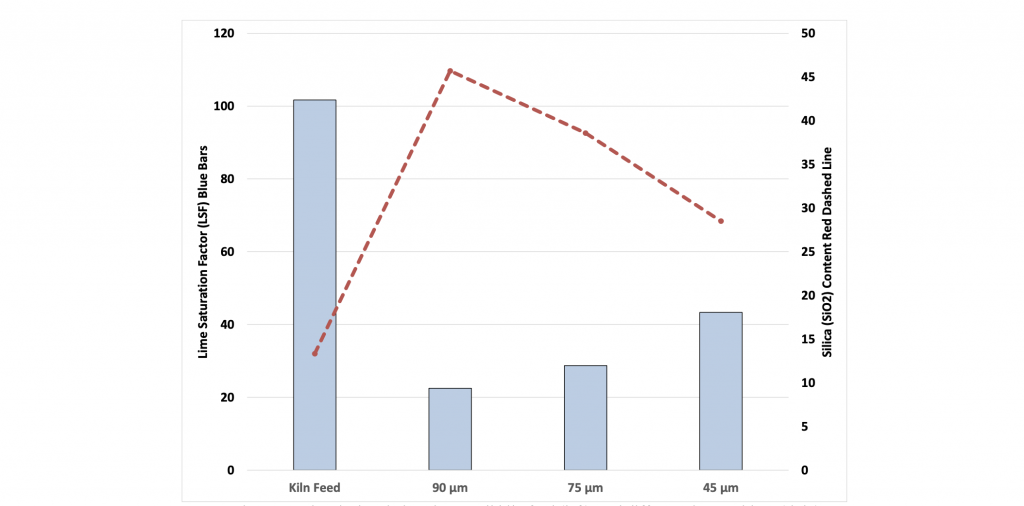
Figure 1: Chemical variations in overall kiln feed (left), and different sieve residues (right)
Here we see that the sizing of the materials is important. Oversized particles of limestone, and especially quartz, will require higher reaction temperatures. Higher reaction temperatures mean more heat leaving the kiln and precalciner in the latent heat of clinker and exhaust gases.
C. Thermal Efficiency
The raw materials need to be heated to the reaction temperature in order to transform them into the new compounds. The conversion temperature is typically between 1425 – 1475 degrees C. At this temperature, the silica will react with the lime to form the calcium silicates. Higher temperatures may be needed when the raw mix is coarser.
Once heated to the clinkering temperatures, the materials combine to form the clinker which is a lava like material that is partially molten. The total heat required for the reactions is roughly half of the total heat consumption for producing the clinker. The remaining heat is lost due to the system inefficiencies.
The thermal efficiency of the process is expressed as the heat required for the chemical reactions divided by the heat consumed in the manufacturing process. The thermal efficiency of clinker production in modern plants is typically above 50 %. This is well above the thermal efficiency of the most efficient gas fired automobiles or thermal power plants today.
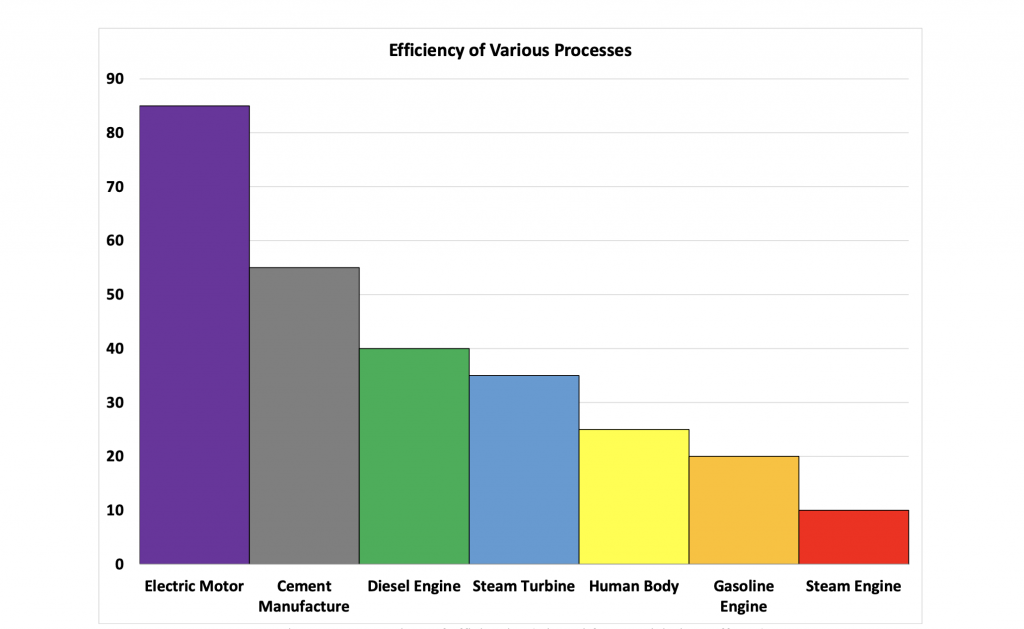
Figure 2: Comparison of efficiencies (adapted from explainthatstuff.com)
The majority of heat leaves the process in two forms: the heat contained in the combustion gases and the heat contained in the clinker. The heat leaving in the exhaust gases is partially reclaimed in the preheater, while the heat leaving in the clinker is partially recuperated in the clinker cooler. Some heat is lost through radiation from the surface of the equipment, most notably the kiln. Some heat loss is required in the combustion zone of the kiln to ensure that a clinker layer known as the “coating” blankets and protects the refractory. Radiation losses are, however, a small portion of the total heat requirements of the system. Radiation losses are typically fixed by the equipment design and choice of insulating materials and not under operator control.
Therefore, the two most important elements in determining the system thermal efficiency are the operation of the clinker cooler and the operation of the preheater. Both pieces of equipment are designed to recuperate some of the heat that is released and required to drive the reactions.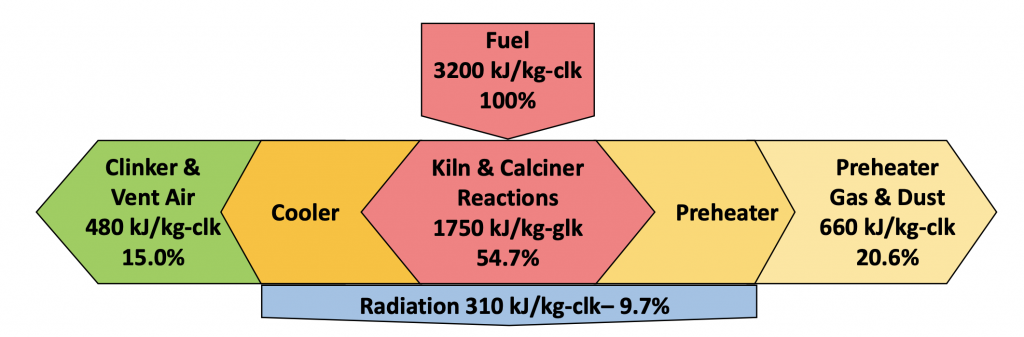
Figure 3: Heat flows in a modern clinkering operation
III. PREHEATER RECUPERATION
The preheater is a straight forward piece of equipment. It is a device used to recuperate heat from the combustion gases and transfer that heat to the raw materials. This cools the combustion gases for eventual cleaning and release to atmosphere while conserving the heat in the process. This is typically accomplished in cement plants through a series of between 4 to 6 cyclone stages.
A. Preheater Design
The heat input into the preheater is a function of the quantity and temperature of the fuel combustion gases and gases released from the chemical reactions. The quantity of the combustion gases are a direct result of the quantity of fuel being combusted. The temperature of the combustion gases is a function of the temperature set point at the exit of the precalciner. The higher the amount of heat going into the preheater, the higher the preheater exit temperature will be.
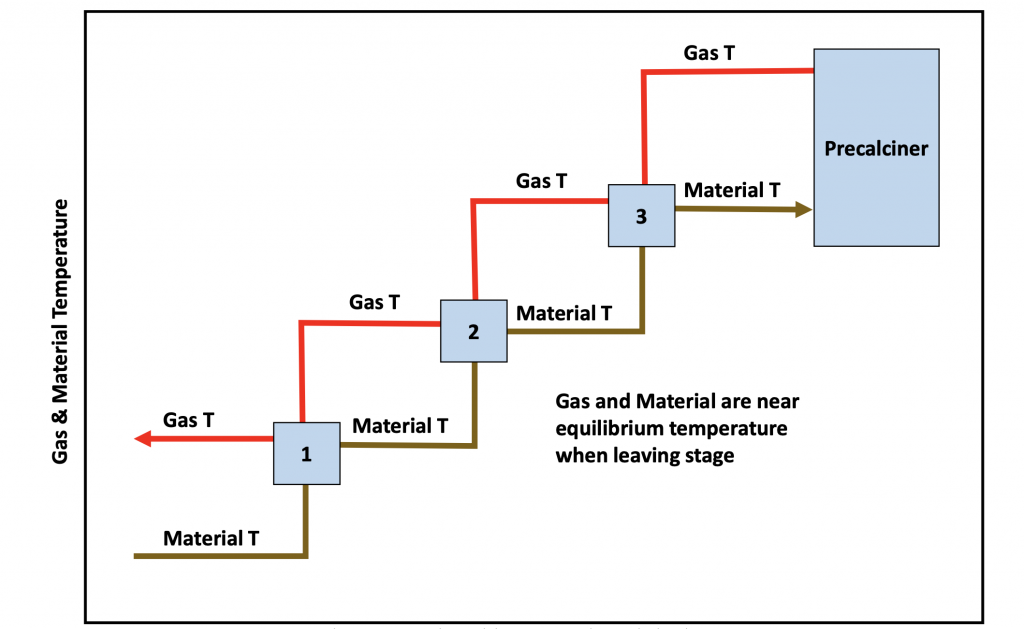
Figure 4: Gas and material temperatures in a typical preheater
The preheater works as a counter current series of co-current heat exchangers. Each individual stage of preheat is co-current as the gas and material travel in the same direction. The material is heated by the gas and both the material and gas reach nearly identical temperatures before being separated by the cyclones. The material then travels counter currently to a lower stage while the gases travel to a higher stage.
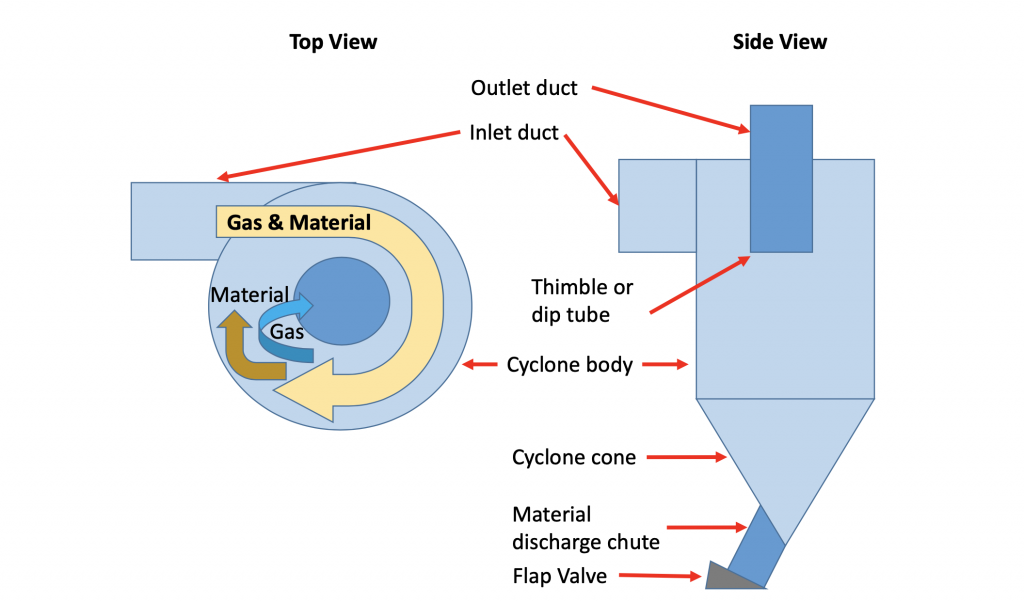
Figure 5: Basic elements of a typical cyclone in a preheater tower
The only moving part in the preheater is the flap or tipping valve, which acts as an airlock between stages. Assuming the tipping valves are working properly, the preheater will deliver a fixed percentage of recuperation of the heat in the combustion gases. That is not to say that the preheater efficiency is always the same, as it can be affected by many factors. However, over short time periods, the preheater efficiency is constant unless / until physically modified.
The factors that affect the overall efficiency of the preheater include: the dispersion of the material into the gas stream and the collection efficiency of the cyclone stages. The better the dispersion of the material into the gas stream, the better the mixing and heat exchange between the gas and material. The main concern here is the design of the splash box. This is the fixed point at which the material is dispersed into the gas stream. The splash box needs to provide a break for the material momentum as well as a good spread of material into the gas stream.
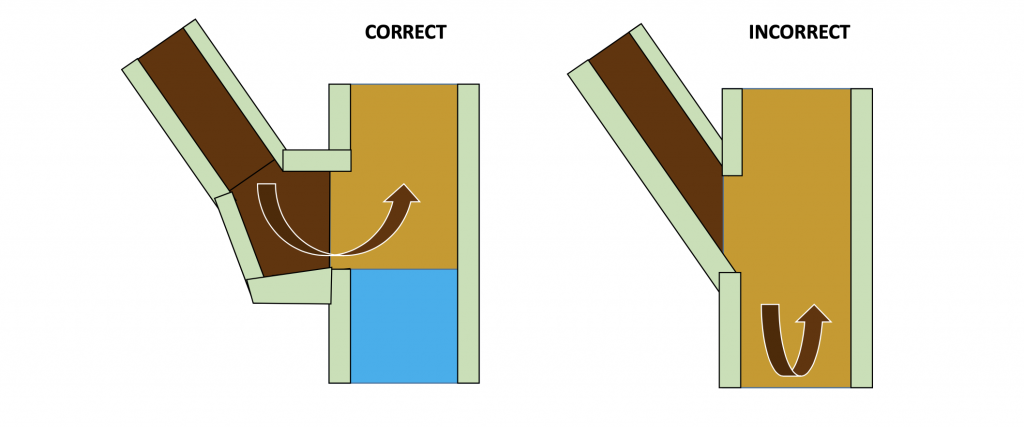
Figure 6: Correct and incorrect splash box design, the arrow indicates the direction of the material
The collection efficiency of the cyclone is fixed by its design. The main factors being: the physical dimensions of the cyclone, especially the diameter and height of the cylinder, and the dip tube or thimble. There is a tradeoff between the efficiency of the cyclone and the size. The higher centrifugal forces required for better separation require a smaller cylinder and higher gas velocity. This in turn requires a higher force to pull the gases through the cyclone, seen as a higher pressure drop. Higher pressure drops require more fan power to pull the gases. This is the classic tradeoff between heat consumption and power consumption.
The most important stage, from a thermal efficiency point of view, is the top stage; the stage where the material enters the system and the gases leave the system. Any material not captured at this point will exit the system, carrying its latent heat with it. This material is subsequently cooled and returned to the system representing a heat loss. The collection efficiency of the top stage should be around 95 %. That is, that 95 % of the material fed to the system will be collected and passed to the next preheater stage. It also means that 5 % of the material will be carried out of the system, taking the latent heat of material with it.
B. Preheater Inlet Temperature
Most modern cement manufacturing facilities utilize the precalcination process for clinker production. In the precalcination process, the basic chemical reactions are separated. The endothermic calcination of limestone is completed at a lower temperature in a stationary vessel called a precalciner (or just calciner). The exothermic combination of calcium and silica to form clinker is completed in a rotary kiln.
The calcination of limestone starts to occur at around 600 degrees C. The calcination process is reversible and therefore the temperature of calcination is dependent on the amount of CO2 in the exhaust gases. That is to say, the higher the level of calcination, the more CO2 that is released and the higher the temperature required to drive the reaction further. Figure 7 demonstrates the relationship between the concentration of CO2 in the gas stream and the temperature required for calcination. The red bubble is the typical operating range for modern precalciners.
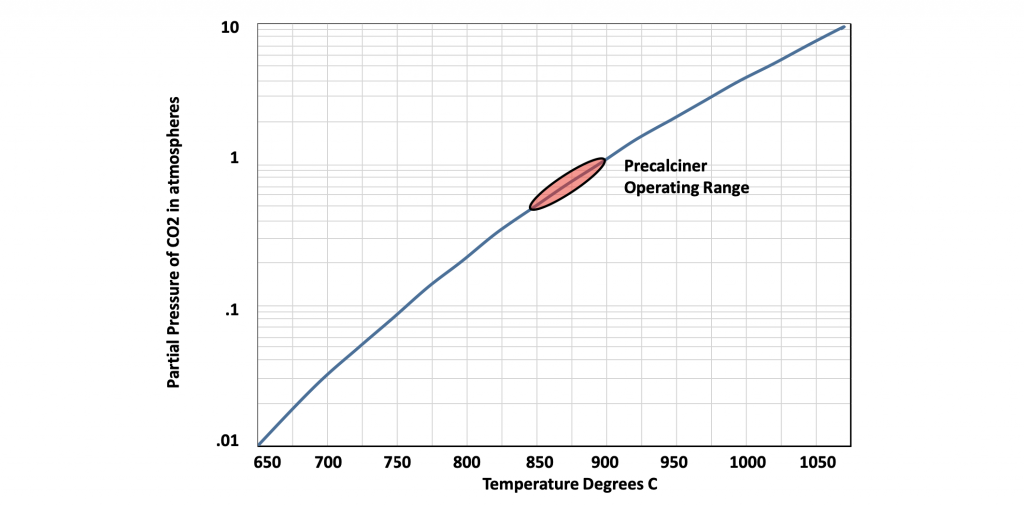
Figure 7: Calcination temperature as a function of partial pressure of CO2 in the gas stream
The benefit of the reversible calcination reaction is that a very uniform level of calcination can be achieved if the temperature and gas flow can be held constant. This, in fact, is one of the basic principles of modern precalciner control. The rate of calcination is controlled by the precalciner exit temperature, which controls the fuel rate to the calciner. A minimum amount of calcination is required to properly prepare the material for the kiln. However, most cement plants run with a higher calcination rate than necessary. Reducing the amount of calcination in the precalciner will reduce the gas temperature into the preheater and reduce fuel consumption.
The preheater recuperates a fixed percentage of the heat in the combustion exhaust gases from the kiln and precalciner. Therefore, the heat losses in the preheater exit gas and dust are directly related to the heat contained in the preheater inlet gas. The higher the fuel consumption, the higher the quantity of combustion gases and therefore the higher the preheater exit temperature. Looking at this in reverse, higher preheater exit temperatures indicate higher system fuel consumption.
IV. COOLER RECUPERATION
The clinker cooler is designed to transport the extremely hot clinker from the kiln while cooling it. Clinker temperatures at the cooler inlet are usually in the range of 1450 degrees C, but this depends on the peak clinkering temperature and burner position. Ambient air is blown into the cooler to reduce the clinker temperature. The cooler is made up of metal parts that would melt if they came in direct contact with the clinker exiting the kiln. Therefore it is important that an insulating layer of cold clinker (or other material) separates the hot clinker exiting the kiln from the exposed metal parts.
It is important to understand that the primary task of the cooler is to transport the clinker away from the kiln. The secondary task of the cooler is to cool the clinker and, in so doing, to recuperate some of the heat contained in the clinker to the process. Heat recuperation is accomplished by using a portion of the heated cooling air as combustion air. Air used for combustion in the kiln is known as “secondary air”, while air used for combustion in the precalciner is known as “tertiary” air. Note: Primary air is the air that enters the process with the fuel.
Coolers typically use twice as much air for cooling as is needed for combustion. The excess air, often referred to as “vent” air, is directed to a filter to remove particulate matter and then vented to the atmosphere.
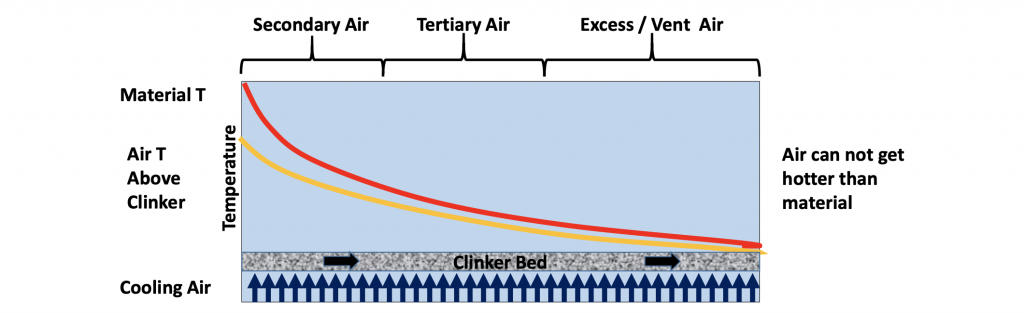
Figure 8: Cooler cross section, material entry on left and exit on right, counter flow of material and air
It is difficult to assess how much recuperation is taking place in the cooler, as the quantity and temperature of the combustion air is difficult to measure directly. However, it is easy to assess the amount of heat that is not recuperated and therefore lost from the system. This is the sensible or latent heat contained in the clinker at the cooler exit as well as the heat contained in the cooler exhaust or vent gases. These two streams are taking heat away from the manufacturing process.
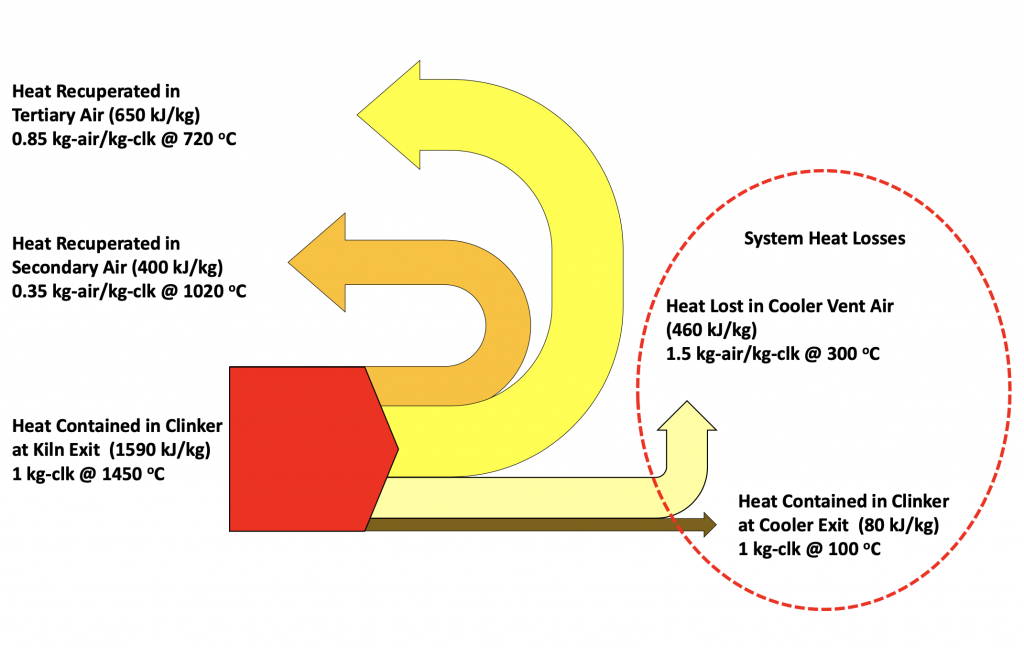
Figure 9: Typical cooler heat balance showing recuperation and losses
The effectiveness of the cooler is a function of many variables. Assuming that the mechanical components are working properly, the main areas of focus are the depth of clinker in the cooler and the amount of cooling air that is being added. The fundamental principle is that more heat is transferred from the clinker to the air with longer clinker-to-air contact time. This means that deeper bed depths and lower air velocities will give the best recuperation.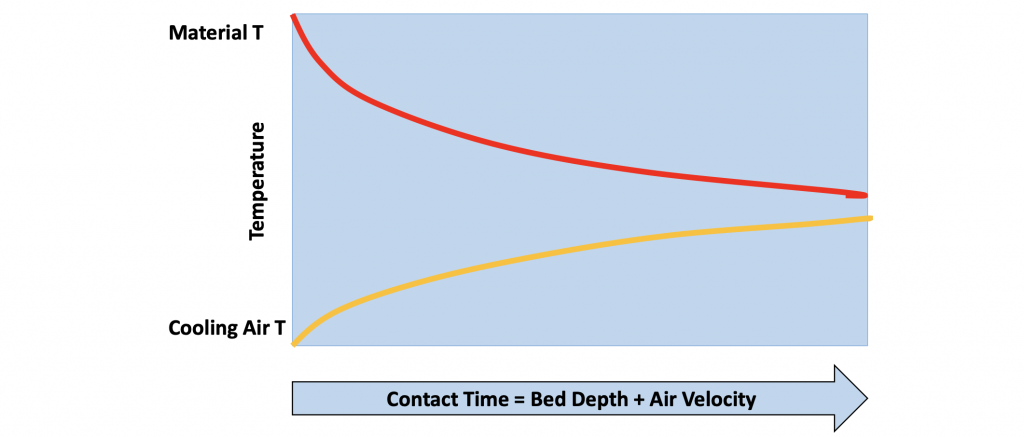
Figure 10: Heat transfer as a function of contact time
However, there are practical limits as low air velocities mean larger coolers which are more costly. Deeper bed depths may require increased fan power to push the cooling air through the clinker. This could mean more expensive and larger fans and more power consumption. The cooler basic design is set by the manufacturer. This determines the approximate bed depth and cooling air velocity ranges that can be used.
Clinker fluidization is one of the main issues in proper cooler operation. Clinker fineness, the weight of the clinker bed, and the cooling air velocity all contribute to fluidization. Clinker fineness is determined by many factors including the chemical composition of the clinker, burning zone conditions, and kiln speed. The discharge of material from the kiln naturally size segregates the clinker as well. Larger clinker particles tend to roll down the clinker bed in the kiln and fall on the down-turning side of the kiln. Finer particles tend to slough off the up-turning side of the kiln as seen in figure 7. When clinker is fluidized it flows like a liquid and the cooler speed no longer controls the material flow.
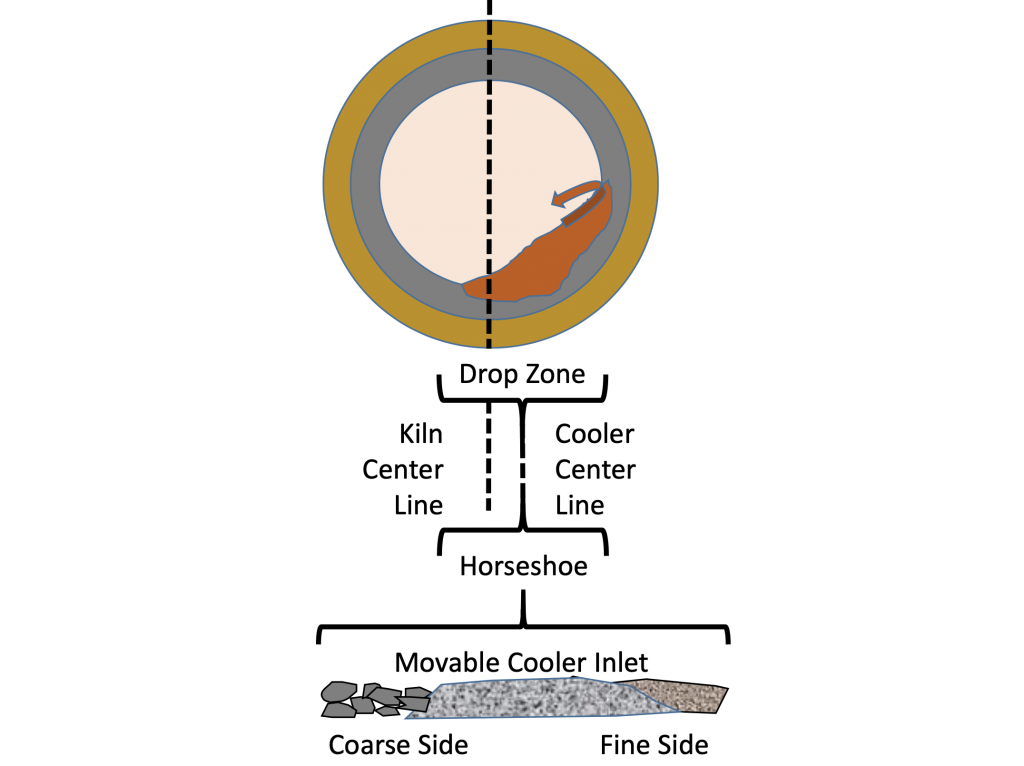
Figure 11: Clinker size segregation caused by rotary kiln discharge
The cooler can recuperate the majority of the heat (+/- 75 %) that leaves the kiln in the clinker. The easiest measurement of the cooler efficiency is to measure the heat lost in the clinker and the vent air. The heat transfer from clinker to air is a function of the contact time, which is driven by the bed depth and air velocities. Higher air velocities therefore reduce heat transfer efficiency and can cause fluidization of the clinker as well. High cooler air velocities is a common problem in the industry. Operators often react to “hot” clinker at the cooler exit by adding more cooling air, which is not always the solution.
V. STABILITY
The system stability has an impact on thermal efficiency. This impact can be manifested in many ways. The main areas to look at are the kiln feed stability, the fuel delivery stability and the burning zone stability. The kiln feed stability is often expressed as the standard deviation of the kiln feed chemistry based on either the lime saturation factor or the expected tricalcium silicate (C3S) content of the clinker. Both factors use different equations but tend to track each other. High standard deviations in these factors indicate chemical instability in the system.
As seen above, the main driver for the heat of reactions is the amount of limestone in the raw mix. The external cement specifications and internal quality standards generally fix a target amount of lime (CaO) in the final product. This target amount directly relates to the heat of reaction to calcine the limestone. When the quantity of limestone varies, the heat required to calcine the limestone will vary. As all limestone needs to be converted to lime, the heat requirement will be a function of the peak quantity of lime in the raw mix and not the average amount. When the limestone fluctuates to a lower level the system will probably not react fast enough and the clinker could become over prepared and less reactive.
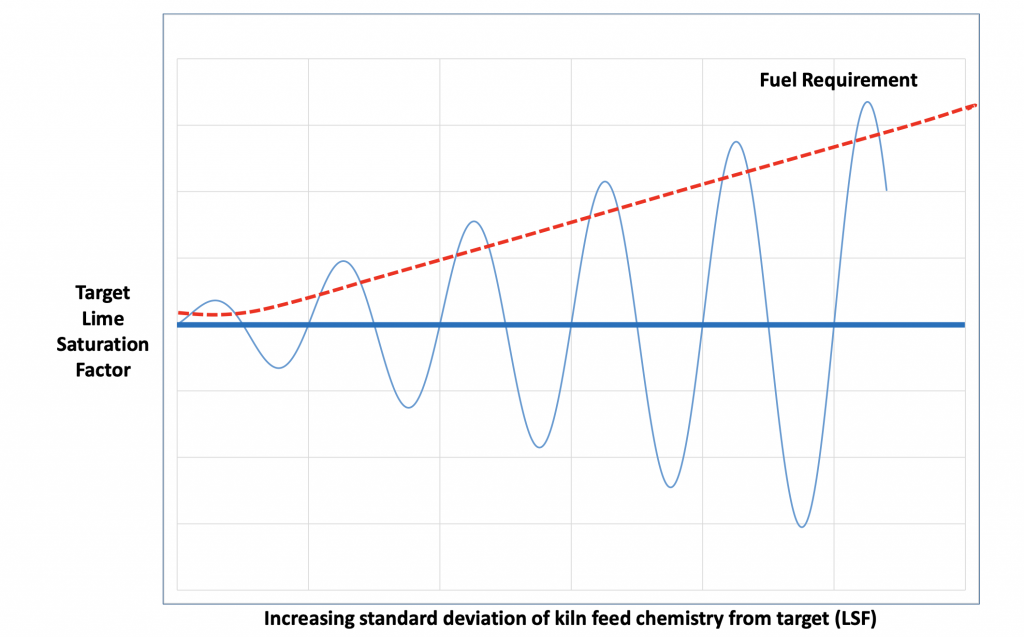
Figure 12: Impact of chemical instability on flame temperature and heat consumption
One of the large destabilizing factors in chemistry is the size distribution of the various components in the dust leaving the preheater tower. The preheater utilizes cyclones to separate the material from the gases, which rely on gravitational and centrifugal forces for separation. The exhaust gases and dust enter the cyclone body in a tight swirl which moves the heavier particles to the outside wall of the cyclone where they tend to fall by gravity down into the cone section. The finer particles stay entrained and leave the preheater in the gases. The harder to grind raw materials tend to be the coarsest particles, while softer raw materials concentrate in the finer particles. So plants that use sand as a raw material (see figure 1), tend to have their silica fraction more in the coarse side while plants that use clay as a source of silica may have more limestone in the coarse particles.
Either way, when the fine dust leaving the preheater differs in composition from the raw feed chemistry there are often chemical instabilities in the system. One quick measure of the potential for chemical instability is to compare the chemistries of the system dust when the raw mill is not in operation to the normal kiln feed chemistry. Often, problems ensue when there is more than a ten percent difference between these values (expressed as either LSF or C3S). Special attention needs to be paid to how the filter dust is returned to the system when these differences occur.
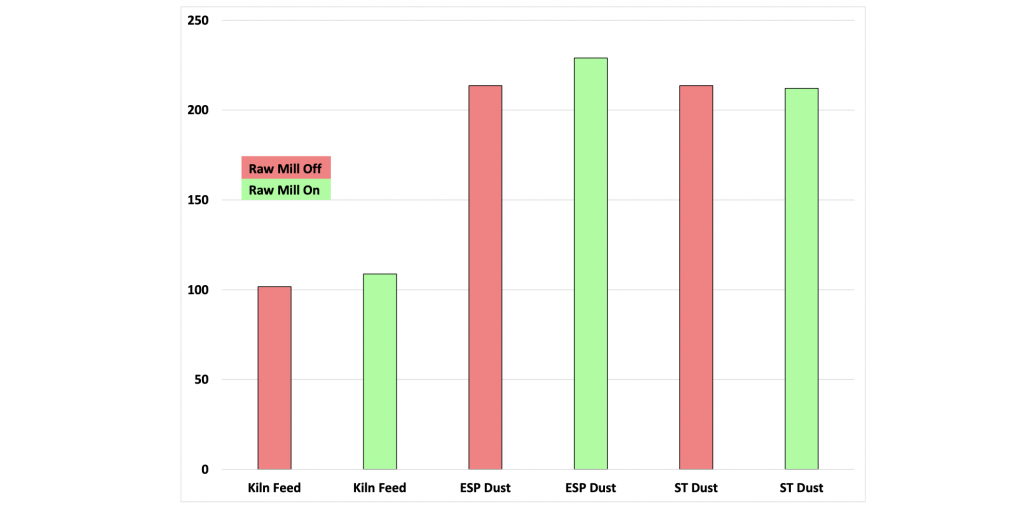
Figure 13: Plant lime saturation values for kiln feed, precipitator and spray tower dust with the raw mill in and out of operation
Chemical stability should be tracked through-out the process, starting with the incoming raw materials. The standard deviations of the chemistry of the raw mill product, preheater feed material, kiln feed material (“hot meal”), and clinker should all be measured and compared. The chemical stability should improve as the process progresses. That is, the clinker should be more stable than the kiln feed, etc. Chemical stability is the best indicator of product stability and a key component in optimizing thermal efficiency.
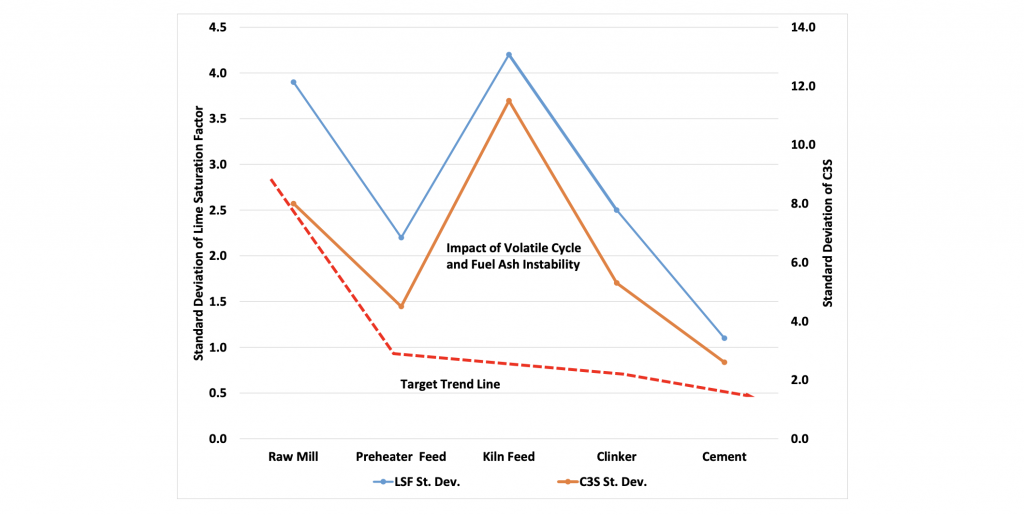
Figure 14: Desired chemical consistency (red dashed line) versus actual plant data
VI. SUMMARY AND CONCLUSIONS
The chemical characteristics of the raw materials determine the total heat of reaction required. This is the starting point for determiningthermalefficiency. Thechemistryoftherawmixisusuallysetbyproductspecificationsandindustrypracticesand tends to be fairly constant across the industry. The physical characteristics of the raw mix will determine the necessary reaction temperatures. The required reaction temperature is the starting point for the recuperation of heat in the cooler and preheater. The total amount of heat recuperated will determine the thermal efficiency of the process.
The system thermal efficiency is determined primarily by the heat recuperation in the cooler and preheater. The cooler is the more difficult to master as the inputs of clinker rate, size and temperature are variable. The cooler’s effectiveness in heat recuperation is a function of many factors starting with the design of the cooler. The primary controls in the operator’s hands are controlling the cooler speed and the cooling air quantity to maximize air to clinker contact time.
The most common problem with coolers is high cooling air velocities causing fluidization problems. Cooler recuperation effectiveness is manifested in the clinker and vent air temperatures leaving the cooler. For modern coolers, the clinker should exit the cooler at around 60° C higher than ambient temperature and the cooler vent temperature should be 300° C or less at the cooler exit. The cooling air quantity should be in the range of 2 kg-air/kg-clinker.
The preheater exerts the second largest influence on the overall thermal efficiency of the system. However, the preheater is a much less complicated device than the cooler. Assuming that all of the parts are working as they should be, the preheater will deliver a fixed amount of recuperation depending on the number and efficiency of the cyclone stages.
Therefore, the heat losses from the preheater are a fixed percentage of the heat entering the preheater. The largest factor then becomes the exit temperature of the gases from the precalciner. Reducing the precalciner exit gas temperature will reduce the percent calcination of the material entering the kiln, but will also improve the system thermal efficiency. The right balance between percent calcination which impacts kiln stability and system thermal efficiency is plant specific. However, it is seen that most plants operate at a higher percent calcination than required.
The stability of the system has the third greatest effect on thermal consumption and efficiency. Instability can come from raw material, chemical and physical fluctuations. One good indicator of process stability is the standard deviation of the kiln feed chemistry. Chemical instability is more likely when the filter dust chemistry with the raw mill off varies by more than 10 % from the normal kiln feed chemistry.
Thermal efficiency is important as fuel costs can make up 15 – 30 % of the total production costs of the cement. Mastering thermal efficiency for a given system requires close attention to the cooler and preheater operation and controlling the system stability. Paying attention to these points will deliver improved system thermal efficiency and a competitive cost advantage.
References
[1] Bhatty, J., Miller F., Kosmatka S. and Bohan R., “Innovations in Portland Cement Manufacturing”, Portland Cement Association, 2011
[2] Kline J., and Kline C., “Mastering the Kiln”, International Cement Review, December, 2015
[3] Kline J., and Kline C., “The Modern Cooler”, International Cement Review, November, 2015
[4] Kline J., and Kline C., “Preheater Performance”, International Cement Review, August, 2015
[5] Moore, D. “Thermal Aspects of Clinker Production”, http://www.cementkilns.co.uk/ckr_therm.html
[6] Stanmore B., and Gilot P. “Review—calcination and carbonation of limestone during thermal cycling for CO2 sequestration”, Fuel Processing Technology 86, 2005
[7] “Heat Balances of Kilns and Coolers”, Holderbank Cement Seminar, March, 2001
[8] “The Science of Bicycles”, http://www.explainthatstuff.com/bicycles.html




